Medical Affairs
Design and Writing of Clinical Protocol;Draft and Review Clinical Summary ReportPV and Medical Monitoring
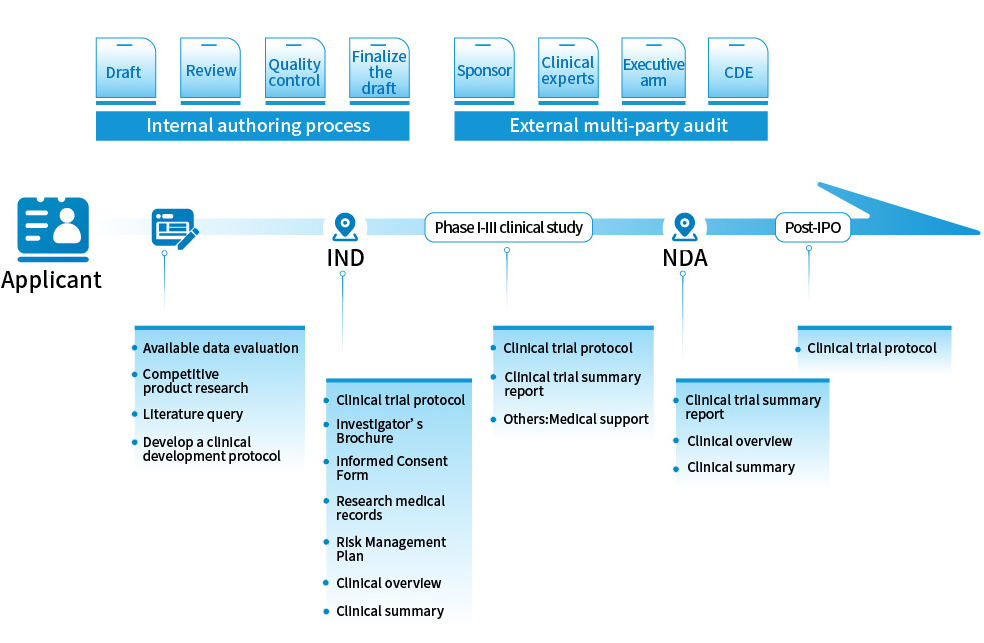
Medical Writing
Write CTD format documents in accordance with ICH M4 requirements, including but not limited to clinical trial protocol and ethical review documents (such as informed consent, case reports, investigator brochure, etc.); Summary documents of clinical safety and efficacy (such as clinical trial review, clinical trial report, clinical trial summary, etc.) and early clinically relevant non-clinical material writing (non-clinical review and non-clinical trial summary, etc.). In addition, it can write and sort out the registration application materials and meeting materials of IND and NDA in accordance with the ICH M4 and CDE review requirements.
Medical Monitoring
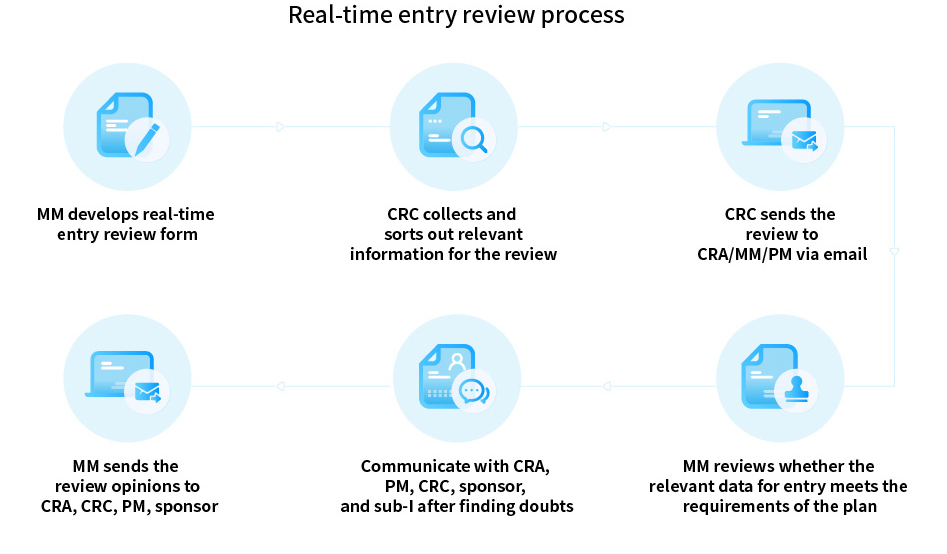
Based on the medical quality control during the implementation of clinical trial protocols, to ensure that clinical trial data are scientific, accurate and in line with medical logic, medical supervision is carried out on the inclusion and exclusion criteria, safety data, efficacy data, drug combination and prohibition, suspected protocol deviation and other information in the clinical trial protocols. Formulate scientific medical monitoring plan according to the actual situation of the project, carry out monitoring orderly according to the monitoring plan through on-site and centralized monitoring. And issue regular monitoring report, timely warning of the problems found in the monitoring process, and develop rectification measures and corrective methods. Ensure the quality of trial data meet the requirements of the protocol.
Medical monitoring-real-time monitoring (Enrollment period)
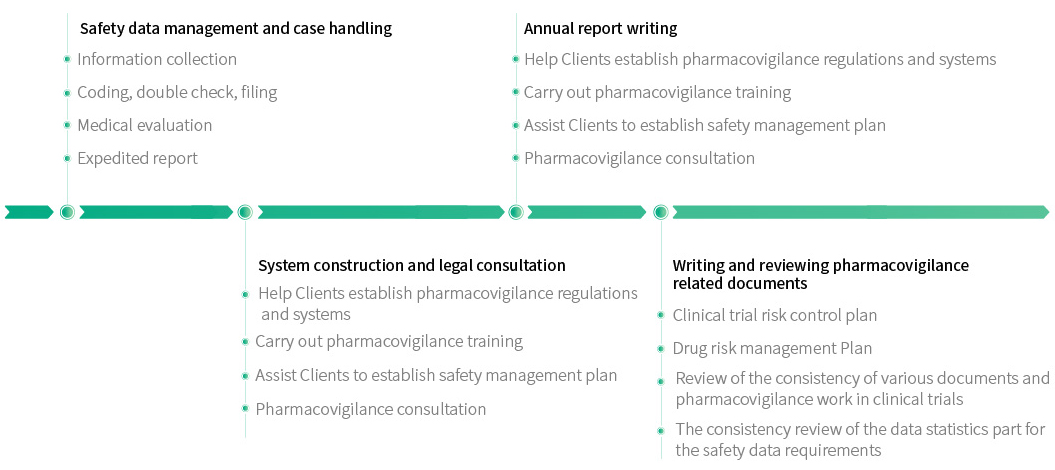
PV
In accordance with relevant national laws and regulations (" Good Pharmacovigilance Practice "), a series of activities in the clinical trial stage, such as the establishment of pharmacovigilance regulations and systems, the processing of individual safety reports, the writing of pharmacovigilance documents, SUSAR reports, etc., are carried out to ensure the safety of subjects in clinical trials and reduce the risks in the process of drug R&D. To provide sufficient basis for evaluating the safety and effectiveness of drugs.