Zifa01 Tablets Project recruits patients with acute symptomatic deep vein thrombosis
Project Introduction
A "multi-center, positive drug, parallel controlled phase II clinical trial to evaluate the effectiveness and safety of Zifa01 tablets in the treatment of acute symptomatic deep vein thrombosis" (NMPA batch number: 2016L01725) is carried out in about 30 research centers with the Chinese Academy of Medical Sciences and Peking Union Medical College Hospital as the team leader. The center is now openly recruiting subjects from the public.
Zifa01 tablets are highly selective and direct inhibitors of factor Xa. They have many advantages such as oral, fixed-dose administration, and a wide therapeutic window. They may be a new effective drug for the treatment of acute symptomatic DVT.
Main criteria
The main selection criteria for participating in this study:
1. Patients aged 18 to 70 years old (including 18 years old and 70 years old);
2. Patients with acute symptomatic deep vein thrombosis who developed symptoms within 14 days before screening and are expected to start using experimental drugs within 14 days (including 14 days) of onset;
3. The result of D-dimer examination during the screening period is greater than the upper limit of the normal range;
The main exclusions for participating in this study:
1. People with venous thrombosis below simple popliteal (not including popliteal);
2. Patients who have undergone or plan to undergo surgery (including surgical thrombus removal, percutaneous mechanical thrombectomy, balloon expansion, stent placement, etc.; only the inferior vena cava filter is allowed to be inserted) to treat venous thrombosis;
3. Patients with confirmed or suspected symptomatic pulmonary embolism;
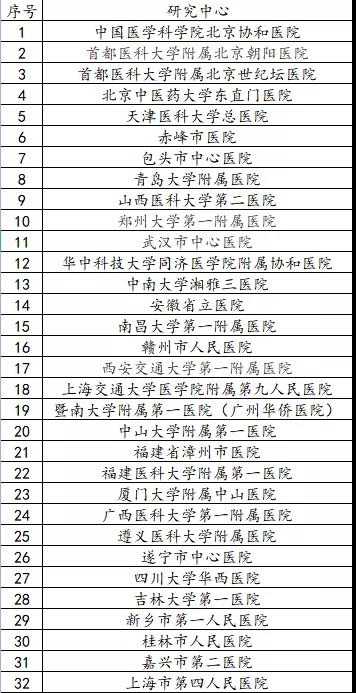
Center list
Sincere invitation
If you are checked by the research doctor and meet the enrollment requirements and you voluntarily participate, you will receive the research drugs provided by the sponsor and related examinations provided by the hospital. We will comply with the requirements to keep your personal information strictly confidential.
Scan QR code to sign up



