Good news for patients with insomnia! Short-term insomnia clinical trial recruitment
Dear patients:
Hello!
The "Multi-center, randomized, double-blind, placebo, parallel controlled Phase III clinical trial to evaluate the effectiveness and safety of doxylamine succinate tablets in the treatment of short-term insomnia" initiated by Nanjing Jiqun Pharmaceutical Technology Co., Ltd., is now openly recruiting subjects from the society. This clinical trial is to verify the safety and effectiveness of doxylamine succinate tablets in improving sleep.
Doxylamine succinate tablets were first marketed in the United States in about 1978, and have been subsequently marketed in Europe and the United States and other countries, and they are OTC drugs abroad. It is now launching clinical verification trials for listing in China. This trial has been approved by the Clinical Trial Notice (CYHS1900169) and the Ethics Committee of the National Medical Products Administration.

The main selection conditions for participating in this trial:
1. Age 18-65 (including boundary value), no gender limit;
2. Those who meet the diagnosis of short-term insomnia;
3. Able to cooperate to complete 2 sleep monitoring (2 consecutive nights each time for sleep monitoring at the research center);
4. During the experiment, the subjects agreed to observe the daily bedtime of about 21:00~24:00 in the evening, and stay in bed every night for about 7~9 hours;
5. Volunteer to participate in this study, cooperate with the research doctor to complete relevant examinations and fill in relevant scales

If you meet any of the following criteria, you will not be able to participate in this study:
1. Past or present asthma attacks, severe emphysema or chronic bronchitis, glaucoma, peptic ulcer with pyloric or duodenal obstruction, dysuria caused by prostatic hypertrophy, bladder neck obstruction.
2. There are lifestyles that may interfere with sleep: such as travel across time zones or shifts (night shifts and day shifts) in the last two weeks.
3. Special professionals who need to operate machinery during the test, such as professional drivers, high-altitude workers, etc.
4. Female subjects who are in pregnancy or lactation.

See the picture below for the research center:
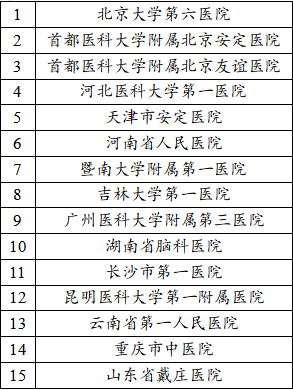
If you meet the enrollment requirements after the study doctor's examination/inspection, and you voluntarily participate, the sponsor will provide experimental drugs and related inspection fees. At the same time, we will keep your personal information and test information strictly in accordance with laws and regulations. Thank you again for your contribution to the clinical cause of Chinese medicine! According to your location, you can register for the nearest research center. After submitting the registration, the researcher will contact you as soon as possible, thank you!

Scan the QR code and fill in the registration


