National Hypertension Day | Let's talk about the research and development status of hypertension drugs
Today is National Hypertension Day.
China has a large population of hypertensive patients, currently exceeding 270 million yuan. In 2013, the direct economic burden caused by hypertension reached 210.3 billion yuan, accounting for 6.61% of the total health costs in China. It causes a huge national medical burden.
According to the 2018 Revision of Chinese Guidelines for the Prevention and Treatment of Hypertension, the important risk factors of hypertension in Chinese population mainly include: high sodium, low potassium diet, overweight and obesity, excessive drinking, long-term mental stress and age, family history of hypertension, physical inactivity, diabetes, dyslipidemia and other causes.
As a part of the pharmaceutical industry, today we simply revisit the classification and research status of hypertension drugs.
At present, the number of antihypertensive drugs that have been marketed worldwide is large, mainly based on the traditional antihypertensive mechanism of action. FDA represents the international standard and level of new drug research and development. By combing the antihypertensive drugs approved by FDA over the years, it can be seen that the peak period of research and development of conventional antihypertensive drugs is in the 1980s ~ 1990s, and a total of 40 antihypertensive drugs have been approved during the past 20 years. Before the 1980s, because basic medical research is still in the accumulation stage of hypertension, it is the storage period of antihypertensive drug research and development; however, after the 1990s, conventional antihypertensive drugs gradually faded out of the hot field of new drug research in the 21st century.
The reasons for the slowdown in the study of antihypertensive drugs may be mainly related to the slow progress in the basic medical research of hypertension, increased cost of new drug development, and reduced proportion of clinical benefits to the market.
Table 1 Development history of hypertension drugs
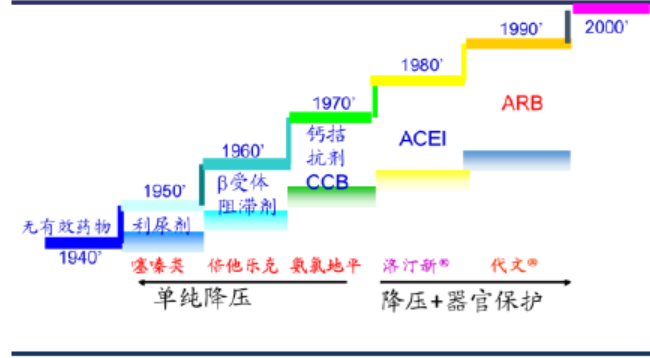
After decades of development, antihypertensive drugs have evolved from early thiazide diuretics, β-blockers, calcium antagonists, and angiotensin-converting enzyme inhibitors (prolides) to angiotensin receptor blockers (sartans). Sartans have become the most widely used antihypertensive drugs due to their more thorough antihypertensive effect, fewer side effects, long efficacy, and ability to be used in combination with other sartans.
Table 2 Classification, mechanism and deficiency of antihypertensive drugs
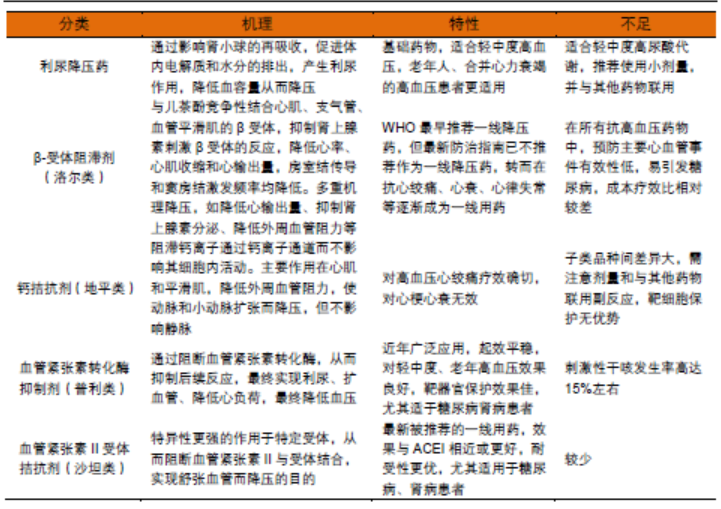

Because hypertension is often associated with other cardiovascular diseases, the condition is very complex, patients need long-term medication, while a variety of antihypertensive drugs are taken at the same time, exacerbating the compliance of patients with medication. Based on the current situation and clinical needs of antihypertensive treatment, the current research and development of antihypertensive drugs mainly develops in three major directions: new drugs, sustained and controlled release technology, and fixed compound preparations.
3. New drugs
Since the information of phase I clinical trials is rarely published, only the drugs in phase 2 and 3 clinical trials are listed in this part.
1. phase III clinical trial drugs
At present, the number of drugs directly used to reduce blood pressure is small, and there are few drugs in phase III clinical trials; sparsentan is an oral bidirectional ACEI and has a highly selective Eta receptor blocking effect. Compared with irbesartan, this product can significantly reduce the degree of proteinuria. TRC-150094 is a silent information regulator 2 homolog 1 (SIRT1) agonist that is mainly associated with metabolic diseases and is not mainly used in hypertension but has the potential to be used in the comprehensive treatment of hypertension.
2. phase II clinical trial drugs
There are a large number of drugs in phase II clinical studies: Firibastat is a (first-in-class) hypertensive drug that slows down the production of angiotensin III and is the first BAPAI candidate selected by Quantum Genomics. This drug is a drug that delivers EC33 products in the brain, which is a selective and specific aminopeptidase A inhibitor, thereby preventing the production of angiotensin III and exerting a central antihypertensive effect by inhibiting aminopeptidase without affecting peripheral angiotensin system (RAS) activity. PL-3994 is an atrial natriuretic peptide agonist that can synergistically act with ACEI to exert its antihypertensive effect. It is expected to be used in combination with antihypertensive standard therapy in clinical practice. Praliciguat (IW-1973) is an oral, soluble guanylate cyclase (sGC) stimulator for the potential treatment of diabetic nephropathy and heart failure with preserved systolic function (HFpEF). It can improve vascular and metabolic function and reduce the inflammation and fibrosis associated with these diseases. Although it has antihypertensive effect, it is mainly used for the treatment of other cardiovascular diseases. LHW-090 is a membrane metallo-endopeptidase inhibitor that has shown significant antihypertensive effects in clinical trials and has no significant adverse effects compared with the placebo group. Rostafuroxin is a sodium-potassium channel-converting enzyme inhibitor that also affects the Src signaling pathway and has both cardio-cerebral and renal protective effects while lowering blood pressure. Vasomera study code PB-1046 has good biological activity, and only subcutaneous injection once a week is required in clinical trials to exert good clinical effect.
Controlled release technique
In order to overcome the shortcomings of some antihypertensive drugs with short half-life and multiple doses, many companies have developed sustained and controlled release antihypertensive drugs, such as felodipine, which is made into a sustained-release preparation, and patients take the drug less often per day.
Fixed combination
Fixed compound preparations have multiple advantages: several drugs act synergistically to improve antihypertensive efficacy; reduce the single dose of each drug, reduce its side effects, or make some side effects offset each other, and the patient's blood pressure decreases more steadily. At the same time, the fixed combination helps to increase patient compliance compared to multiple single drugs used together. Over the past 15 years, many fixed-dose combination drugs consisting of two or more drugs have been marketed worldwide. Amlodipine is one of the preferred drugs in all two-drug or three-drug combination products.
However, due to the safety requirements of cardiovascular drugs and the many projects required to be carried out in clinical trials, the R & D cycle for the development of new antihypertensive drugs is relatively high, with high cost and high risk-benefit ratio. To enter this field, pharmaceutical companies should be very cautious and carefully carry out early investigation and project approval.
References
[1] Haitong Securities Research Report Jibel: "Research, Production and Sales" Integration, Multi-product Coexistence
[2] The second half of the consistency evaluation of the special report of the pharmaceutical industry of Ping An Securities opened: early birds eat insects
[3] Priority review of Industrial Securities has accelerated, and the industry has long met Ganlin
[4] Feng Yidong, Feng Hanlin. Progress in Research and Development of Antihypertensive Drugs [J]. Chinese Journal of Modern Drug Application, 2020, v.14 (04): 234-238.
[5] Ma Qianli. The research and development of antihypertensive drugs has shown a trend [N]. China Medical Journal, August 5, 2019 (004).


