New rules, new starting point, solution to the innovation number of the pharmaceutical industry look here! Yiduzhengkang Meet You: The 4th China Forum on Innovative Drugs and Editorial Board Meeting of China Journal of New Drugs (October 16-18, 2020)
In October, the "2020 Fourth China Forum on Innovative Drugs and Editorial Board of China Journal of New Drugs" sponsored by China Journal of New Drugs will be held in Beijing Yichuang International Convention and Exhibition Center. As a co-organizer, Yeedozencom sincerely invites you to attend the event (October 16-18, 2020)!
 In October, the "2020 Fourth China Forum on Innovative Drugs and Editorial Board of China Journal of New Drugs" sponsored by China Journal of New Drugs will be held in Beijing Yichuang International Convention and Exhibition Center. As a co-organizer, Yeedozencom sincerely invites you to attend the event (October 16-18, 2020)!
In October, the "2020 Fourth China Forum on Innovative Drugs and Editorial Board of China Journal of New Drugs" sponsored by China Journal of New Drugs will be held in Beijing Yichuang International Convention and Exhibition Center. As a co-organizer, Yeedozencom sincerely invites you to attend the event (October 16-18, 2020)!
Sharing Yeedo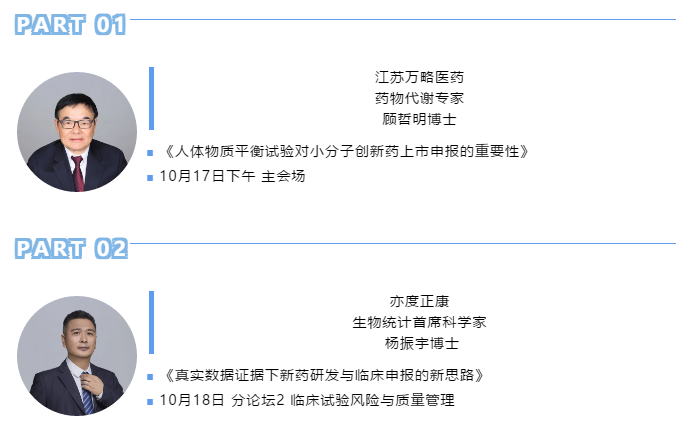 Meeting introduction:
Afternoon of October 17
October 18, all day
9:30-15:30 on October 18
9:30-15:30 on October 18
9:30-15:30 on October 18
Meeting Tips:
About Yeedozencom
Meeting introduction:
Afternoon of October 17
October 18, all day
9:30-15:30 on October 18
9:30-15:30 on October 18
9:30-15:30 on October 18
Meeting Tips:
About Yeedozencom

Sharing Yeedo

Conference name: The 4th China Forum on Innovative Drugs and Editorial Board of Chinese Journal of New Drugs in 2020
Conference time: October 16-18, 2020
Venue: Beijing Yichuang International Convention and Exhibition Center
Conference theme: New rules and new starting point
Supporting institutions: Center for Drug Evaluation, CFDA, Center for Drug Inspection, National Institutes for Food and Drug Control, Chinese Pharmacopoeia Commission, China Pharmaceutical Group Co., Ltd., Beijing Economic and Technological Development Zone
Cooperation units: China Health Media Group, China Pharmaceutical Industry Research Institute, China Pharmaceutical Association and China Pharmaceutical Industry Information Center
Meeting Agenda
October 16: Conference check-in
Afternoon of October 16:
Pre-conference:
Forum on Drug Device Integration: Forum on Innovative Medical Oncology
Chairman: Academician Wang Guangji
Chaired by: Shi Yuankai (Cancer Hospital, Chinese Academy of Medical Sciences)
Speech by academician: current situation and prospect of cancer treatment
Academician Wang Guangji
Progress in cancer drug innovation and medical innovation
Shi Yuankai (Cancer Hospital, Chinese Academy of Medical Sciences)
Selection and expedited review of new overseas drugs urgently needed for rare diseases in clinical practice
(Center for Drug Evaluation, CFDA)
Mechanism and prospect of tumor electric field therapy
Round table discussion:
How to improve the accessibility of innovative cancer therapies (registration access, in-hospital access and payment access), medical devices with conditional approval and exemption from clinical trials, and how to use domestic real-world data for approval
(Xu Binghe, Wang Yongjun, Yang Li, Center for Drug Evaluation, Center for Medical Device Evaluation, National Institutes for Food and Drug Control)
Chairman: Academician Wu Yiling
Chaired by Wei Shuyuan (China Pharmaceutical Industry Research Institute)
Opportunities and challenges for new drug research and development
Academician Wu Yiling
Selection and Priority Review of Breakthrough Therapy Drugs
(Center for Drug Evaluation, CFDA)
Recent progress in clinical diagnosis and treatment of COVID-19
Liu Yaning (Chinese PLA General Hospital)
Research and development of antibody-drug conjugate (ADC) and bispecific antibody drugs in China
Tao Weikang (Jiangsu Hengrui Medicine Co., Ltd.)
Drug Innovation and Intellectual Property Protection
Guo Liang (Zhongge (Beijing) Certification Co., Ltd.)
Importance of human mass balance test for marketing application of innovative small molecule drugs
Dr. Zheming Gu (Jiangsu Wanlue Medical Technology Co., Ltd.)
Round table discussion: priority review and breakthrough treatment of new drugs
Sub-forum 1. R & D and clinical study of new drugs of traditional Chinese medicine
Chairman: Academician Huang Fuqi, Academician Tong Xiaolin
Chaired by Liu Qingquan (Beijing Hospital of TCM, Capital Medical University)
Ye Zuguang (China Academy of Chinese Medical Sciences)
New drug research and development in anti-epidemic practice
Academician Zhang Boli
Discussion on the Development of Traditional Chinese Medicine from the Practice of War Epidemic of Traditional Chinese Medicine
Academician Tong Xiaolin
Thinking on the registration of new drugs of traditional Chinese medicine based on the new crown epidemic situation
Liu Qingquan (Capital Medical University Beijing Hospital of Traditional Chinese Medicine)
Encouragement policies for the research and development of new drugs of traditional Chinese medicine
(Department of Registration, CFDA)
Progress in statistical design in the study of new drugs of traditional Chinese medicine
Yinping (Huazhong University of Science and Technology)
Application of real-world evidence in the research and development of new drugs of traditional Chinese medicine
(Center for Drug Evaluation, CFDA)
New R & D strategies and methods of innovative drugs of traditional Chinese medicine with targeted network module
Wang Zhong (China Academy of Chinese Medical Sciences)
Problems and Thinking of Hospital Preparations in the Research and Development of New Drugs of Traditional Chinese Medicine
Chen Xu (Beijing Food and Drug Administration)
Design and implementation points of pharmacoeconomic evaluation of Chinese patent medicines
Zhu Wentao (Beijing University of Chinese Medicine)
Thinking on the R & D and clinical research of new drugs of traditional Chinese medicine based on practical experience
Cui Tianhong (Beijing Qihuang Pharmaceutical Clinical Research Center)
Round table discussion: new opportunities, priority review and hospital preparations for the research and development of traditional Chinese medicines
Sub-forum 2. Clinical trial risk and quality management
First Half:
Chairman: Academician Yang Baofeng, Academician Zhang Lihe
Chaired by Cui Yimin (Peking University Hospital), Wang Rui (Chinese PLA General Hospital)
Clinical needs-oriented drug innovation
Academician Yang Baofeng
Standardized management and encouragement of innovation of human genetic resources in China
(China Human Genetic Administration Office)
Technical Guidelines for Clinical Changes of Marketed Drugs (Draft for Comments)
(Center for Drug Evaluation, CFDA)
Key role of medical top-level design in new drug clinical studies
Dr. Liying Sun (Haijinge Medicine)
Requirements for subject safety protection and trial risk prevention and control in the new version of GCP
Wang Rui (Chinese PLA General Hospital)
Enterprise R & D strategy and innovative design of clinical R & D
Wu Jinzi (Ge Li Pharmaceutical Co., Ltd.)
New ideas for new drug R & D and clinical application under real-world evidence
Dr. Zhenyu Yang (Chief Scientist, Biostatistics, Beijing Yiduzhengkang)
Risk control considerations in the design of clinical protocol
Dr. Hualong Sun (Colin Likang)
How to prevent safety risks in clinical trials of anticancer drugs
Yan Zhao (Chinese Anti-Cancer Association)
Clinical trial management in the post-epidemic era
Yang Yong (Beijing Borun Sunshine Technology Co., Ltd.)
Round table discussion: clinical trial risks, CDMO
Sub-forum 3: Forum on Pharmacoeconomics and Medical Insurance
Chairmen: Liu Guoen (Peking University), Hu Shanlian (Fudan University)
Chaired by Wu Jing (Tianjin University), Zhai Saidi (Peking University Third Hospital)
Pharmacoeconomics and new drug research and development
Sun Lihua (School of Business Administration, Shenyang Pharmaceutical University)
Application of real-world data in pharmacoeconomic evaluation
Wu Jing (School of Pharmacy, Tianjin University)
Pharmacoeconomic study in medical institutions
Liu Guoqiang (The Third Hospital of Hebei Medical University)
Application of pharmacoeconomics in drug cost control in medical institutions
Leng Jiahua (Peking University Cancer Hospital)
Introduction of pharmacoeconomic evaluation methods of cancer drugs
Fan Changsheng (Beijing Medical and Health Economic Research Association)
Problems and countermeasures in the payment of medical insurance DRGs
Zheng Jie (Beijing Medical Insurance Center)
Discussion on the access mode of medical insurance for drugs for tumors and rare diseases
Wu Jiuhong (Special Medical Center of Strategic Support Army)
Thinking and exploration of medical insurance pricing methods for drugs with multiple indications in China
Li Hongchao (International Pharmaceutical Business School, China Pharmaceutical University)
Round table discussion: how pharmacoeconomics provides health insurance decision support
Sub-forum 4: Frontiers of Biomedicine
Chairman: Academician Chen Zhinan
Chaired by Ding Sheng (School of Pharmacy, Tsinghua University)
Global R & D trend of biotechnology drugs and countermeasures in China
Academician Chen Zhinan
Development status and supervision of cell therapy industry in China
(Center for Drug Evaluation, CFDA)
Current research status of stem cell therapy worldwide
Lin Xin (Tsinghua University)
Current research and development status of new crown pneumonia vaccine
(Center for Drug Evaluation, CFDA)
Characteristics and immunization strategies of developing new crown vaccines by different technical routes
Wang Huaqing (Chinese Center for Disease Control and Prevention)
Venue: Beijing Yichuang International Convention and Exhibition Center
Address: No.6, Rongchang East Street, Yizhuang, Daxing District, Beijing
Hotel: Beijing Singji Platinum Hotel
Address: No. 12, Ronghua South Road, Daxing District, Beijing
Route:
Capital Airport to Rongchang East Street
Capital Airport Line → Metro Line 10 → Metro Yizhuang Line
Beijing Daxing Airport to Rongchang East Street
Daxing Airport Line → Metro Line 10 → Metro Yizhuang Line
Beijing West Railway Station to Rongchang East Street
Metro line 7 → Metro line 5 → Metro line Yizhuang
Beijing South Station to Rongchang East Street
East Section of Metro Line 14 → Metro Line 5 → Metro Yizhuang Line
Beijing Station to Rongchang East Street
Metro line 2 → Metro line 5 → Metro line Yizhuang
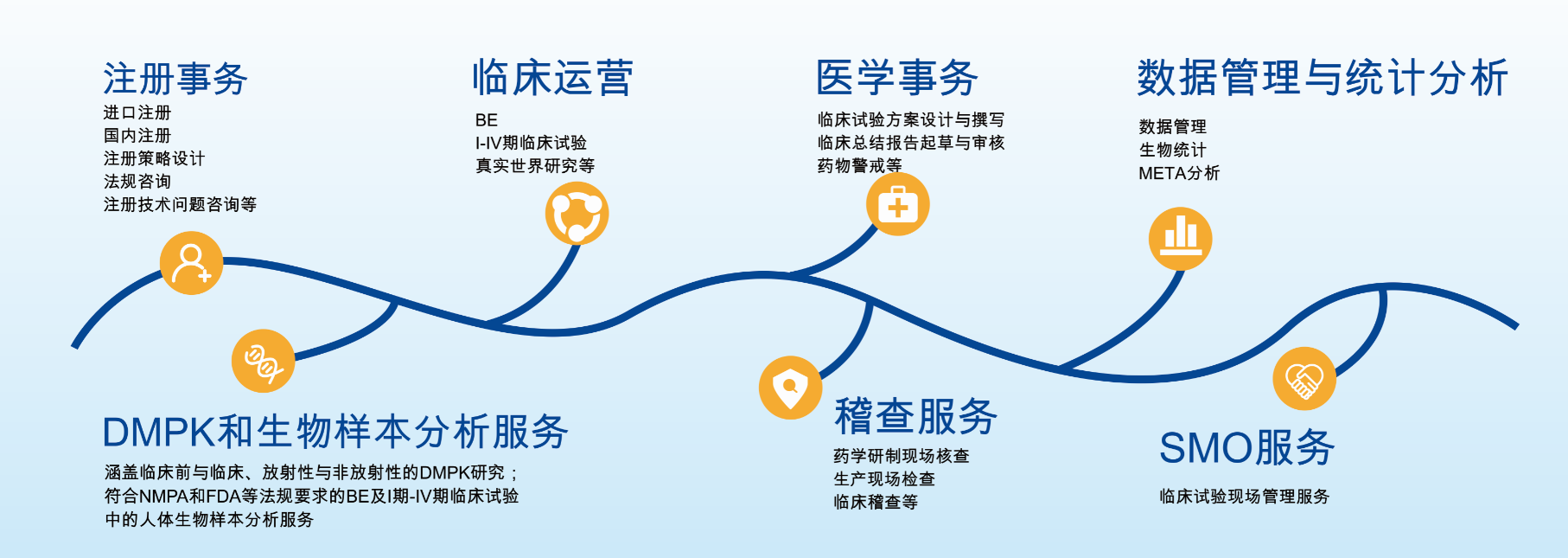
Beijing Yeedozencom Health Technology Co., Ltd. is a leading comprehensive outsourcing service provider for drug R & D in China, committed to providing customers with whole life cycle services from project evaluation, R & D management, clinical trials, registration services to post-marketing research, and is a national high-tech enterprise.
Yeedozencom has its headquarters in Beijing, business unit in Nanjing and service outlets in Tianjin, Guangzhou, Xi'an, Shenyang and Chongqing, covering the mainstream areas of domestic clinical research business. Yeedozencom has an integrated comprehensive service platform. Focusing on clinical research and registration services, DMPK and Biological Sample Testing Center (subsidiary Value Pharmaceutical Services Co., Ltd.), Data Management and Statistics Center (subsidiary Liaoning Yeedo Medical Data Technology Co., Ltd.), SMO On-site Service Center and Medical Software Development Center have been established.
Provide value-added technical services for the whole life cycle of drug R & D

CRO service provider with both depth and temperature
Company address: 9th Floor, Building 5, State Investment Fortune Plaza, No.9, Guang'an Road, Fengtai District, Beijing, China


