News Express – Jiangsu Value Pharmaceutical Services Co., LTD.
Jiangsu Value Pharmaceutical Services Co., LTD. has won full scores in the external quality assessment by National Center for Clinical Laboratories (NCCL, China) for four consecutive years.
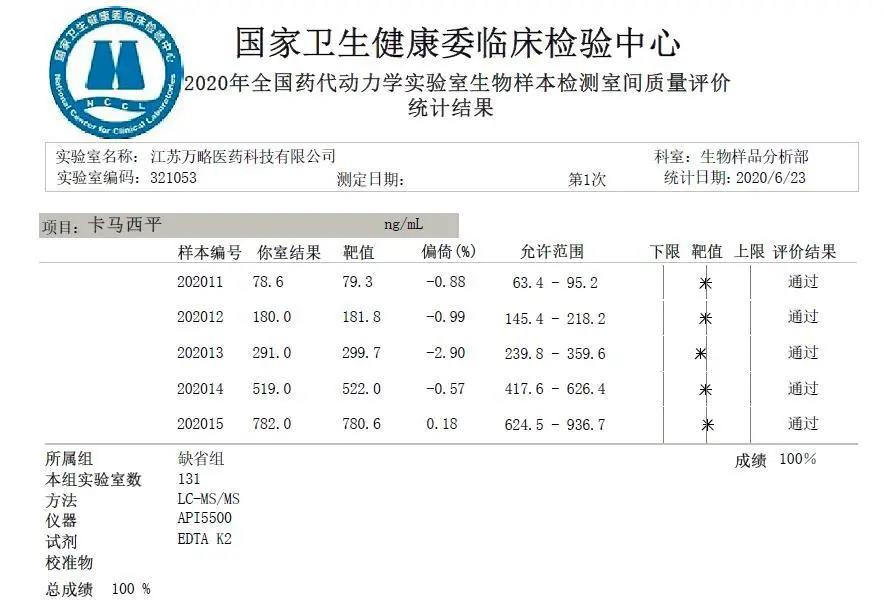
Recently, NCCL published the results of “National External Quality Assessment of National Pharmacokinetic Biological Samples in 2020”, and our biological analysis laboratory has passed the assessment with full scores! This is the fourth full score that Jiangsu Value Pharmaceutical Services Co., LTD. has obtained since 2017 when it began to participate in the external quality assessment by NCCL.
The results of this blind test show that the maximum bias is only 2.90%. Such achievements precisely represent the efforts our biological analysis laboratory that “adheres to science and technology; complies with law and regulations” has made in quality management system. This achievement is also highly consistent with the high ISR test passing rate (nearly 100%) of the biological analysis projects we have completed.
External quality assessment (EQA, external quality assessment) is a crucial means of objectively assessing laboratory testing capacity and sustainability in a quantitative way, identifying interlaboratory difference and increasing the confidence of laboratories and clients. EQA is also an important basis of laboratory quality system certification and supervision. The external quality assessment of national clinical laboratory organized by Center for Clinical Laboratories of the Ministry of Health is the most authoritative standard with the highest domestic recognition for external quality assessment of laboratories in China.

Jiangsu Value Pharmaceutical Services Co., LTD.
Jiangsu Value Pharmaceutical Services Co., LTD. (hereinafter referred to as “Company”) is a contract research organization (CRO) for clinical bioanalysis in accordance with CFDA and FDA regulations, specializing in clinical and non-clinical pharmacokinetics (DMPK) research of new drugs and characterized by low-energy radioactivity (14C, 3H) to establish pharmacokinetics. Established in Nov.,2015, the company is a high-tech enterprise recognized by our country.
The company provides the evaluation of pharmacokinetics and pharmacodynamics (PK/PD) correlation, new drug development and regulatory consultation (including synthesis of 14C / 3H marker, DMPK test design, human 14C test method, establishment of laboratory for biological analysis, etc.) and other services.
The company's clinical biological sample analysis laboratory is committed to meeting the requirements of the CFDA and the FDA GLP regulations; the laboratory adopts electronic management, including one-stop summary output of a verified Watson LIMS laboratory information management system, data auto-transmission from the balance, electronic template for experimental records, experimental plans and electronic data of experimental reports; clinical sample analysis was conducted using modern industrial management, automatic sampling system, SPE and other high-throughput methods to reduce the rate of reanalysis. Most projects have high ISR passing rates, and no projects has failed to pass ISR so far. More than 120 bioanalytical methods for drugs have been established.
The company has established a pioneering methodology system for the study of metabolism of macromolecular drugs in animals such as antibodies labeled with low energy radioisotopes (14C and 3H) and antibody conjugated drugs (ADCs) as well as the study on metabolism/degradation of absorbable medical devices in animals and the current completed type, including PEG crosslinked, polysaccharide, etc. This technique is superior to traditional 125I organization distribution research and gains international recognition.


